Developable strips: from flat to twisted
A robotic metal bending workshop with Dr Efilena Baseta and Marielena Papandreou.
Aluminium 1050-H24
Metal can be purchased accompanied by an alphanumeric code which carries information as to its composition and its treatment before it came to be in your hands. The 1mm thick aluminium sheets used for this workshop had the code 1050-H24, which follows a standard format:
– The first four numbers are the alloy designation of the metal – they tell its chemical composition. The 1000 series is a commercially pure metal, composed of 99.5% aluminium, with 0.4% iron added for conductivity, and 0.2% silicon for hardness. This composition means it has a low mechanical strength, but a high corrosion resistance, high ductility, and a brightly reflective finish. The 3xxx, 5xxx or 6xxx are considered the best for bending, but the 1050 is a good general use sheet, used in light fittings amongst many other things. 1xxx also indicates that the aluminium is non heat treatable.
– The three-character alphanumeric code that follows describes the temper designation of the metal – how hard it is. ‘H’ indicates it has been strain hardened, ‘H2’ that it has been strain hardened more than required then partially annealed to reduce it to its required hardness, and the full ‘H24’ indicates that the Al is at ‘half hardness’ – where H28 is full hardness.
So we may consider that we are dealing with pure aluminium, Al. Like other metals, Al in its elemental form displays metallic bonding, in which the Al ions sit in a lattice of positively charged ions amongst a ‘sea’ of electrons. Each Al atom contributes 3 negatively charged electrons to this sea: 2 from its 2s orbital, and one from the 1p orbital. This gives it a lower, favourable energy state, and a charge of 3+, and the attraction between these areas of charge density create a stronger bonding force than is found with the other metals in the same row of the periodic table (sodium and magnesium). The aluminium ions settle themsleves into a ‘face centered cubic’ crystal lattice: each Al 3+ ion is hexagonally arranged within a plane. Per unit cell, 74% of the space is taken up by Al ions, which is also a contributing factor to the strength of bonding found in elemental aluminium. It is harder, more malleable and more ductile than sodium and magnesium; characteristics which can be observed when we handle it at a more macro level.
As a lattice filled with repeating units of the same atom, metals are in theory isotropic, meaning they display the same characteristics regardless of direction. In reality, however, faults in the lattice or stray heteroatoms can alter these characteristics, and working of the metal also introduces some directionality.
When a material is bent, the deformation comprises of:
- elastic deformation, which rebounds when the bending force is removed. On a molecular level, elastic deformation only stretches the arrangement of the atoms; it does not move them in relation to each other.
- plastic deformation, which remains once the bending force is removed. On a molecular level, the atoms actually shift in position in relation to one another, and a new order is established through which the deformation is maintained.
The ‘grain’ of a metal is a section of the crystal lattice in a particular directional arangement. In a piece of metal there will most likely exist different grains, and between them a grain boundary, which is a section of the crystal lattice with heterogenous arrangements.
When working the metal, strain hardening – as has been done to the 1050-H24 aluminium – will increase the concentration of vacancies and dislocations within the lattice. making further deformation of the metal more difficult. However, the annealing (heating) applied after will lend energy to the atomic arrangement, reducing the number of grain boundaries and homogenising the lattice structure.
When bending the developable strips pf Al 1050-H24 to take them from flat to twisted, its necessary to cause the molecules to shift past one another, so the deformation is plastic and remains in the material once the force from the robotic arm is removed. How much of the force of deformation will be elastic and how much plastic is a question with many parameters, of which the thickness, chemical composition and temper designation of the material form a significant chunk, but by no means all. Buffering the surface of the aluminium, as we did to remove the sharp edges of the milled perforations, also causes plastic deformation in the metal and, interestingly, has the potential to create nanocrystalline surface layers, which inhibit fatigue crack, resist corrosion, and create functional coatings.
Springback
When it comes to bending, springback is an important issue to handle with. It is the relation of the elastic and the plastic deformation mentioned above, which makes the material rebounds to a certain stage. This is mostly somewhere in between the original state and the angles of the fold being made. In this project we want to have a good expectation on how the springback behaves. It has a big effect on the end result of the workshop and how it looks like the model that is actually designed. To calculate this, we have to make the use of a lot different parameters.
The most important metal parameters affecting springback are;
- Elastic modulus
- Strength
- Thickness and shapes material
- Bend radius
To control the springback we introduced a factor of overbending in this project. This is a variable angle which is added to the fold angle. This variable angle should be exactly the same as the springback of the fold. In this project the overbend is used the same for every fold, but every group used their own overbend angle to experiment with the parameters. We asked every group to measure every angle of resulting folds, their desired angle and their overbend. We got data from this which we used to make an overview of springback in averages and extremes.
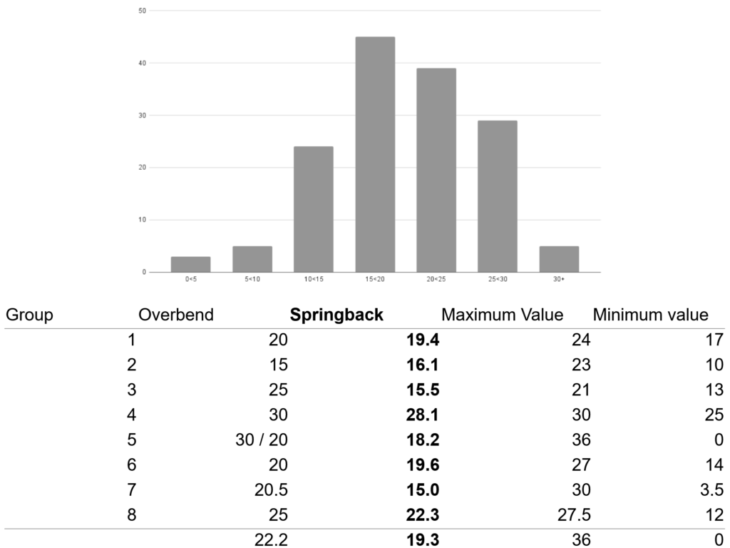
We traced back to the maximum and minimum values and we drawn some conclusions in here:
– The higher the folds, the more springback the material is giving.
– When a bendline is oblique on the material, the springback is also higher. The bendline is longer in this situation but the number of holes to make the bendline is still the same. This means there is more material to be bend.
– The more distance is used between the grippers also resulting to a higher springback. This is caused because the gripper sometimes has to be in an angle to fold the material. In this situation the material around the fold line bends with it and causes a less bend in the bend line.
What we can learn from this for future bend projects is to use different overbend angles on every bend. It is important to use all the parameters to calculate the actual overbend. For instance using a higher overbend angle on higher desired angles. When the bendline is oblique you also need more holes to make the material in every bendline the same or/and use a higher overbend angle.
‘Workshop 1.2: Aluminium characteristics for bending’ is a project of IAAC, Institute for Advanced Architecture of Catalonia developed at Master in Robotics and Advanced Construction in 2021 by students Grace Boyle and Vincent Verster, and faculty: Efilena Baseta and Marielena Papandreou