Crystallization
Introduction:
Crystals are made up of regular repetition of pattern of atoms, or molecules, all alike……. like the regular repetition of pattern of a wallpaper
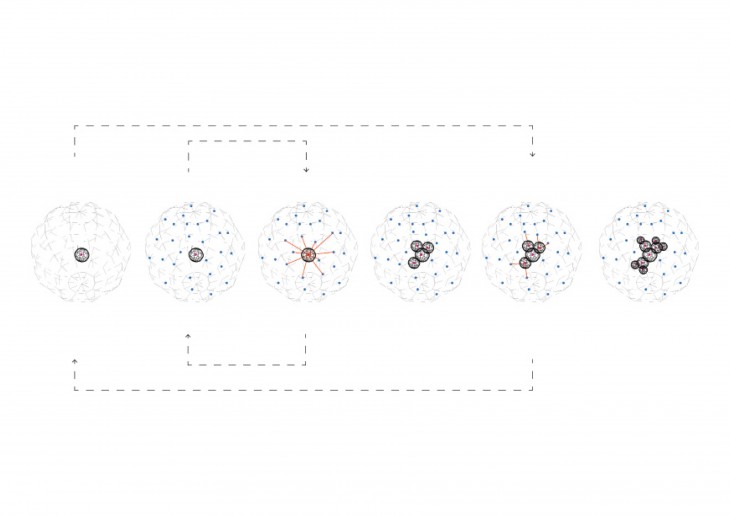
The atoms take up an orderly arrangement, like arranging pennies, as they attach themselves to their surfaces in similar ways because the condition is same in all these faces. and they keep their shape.
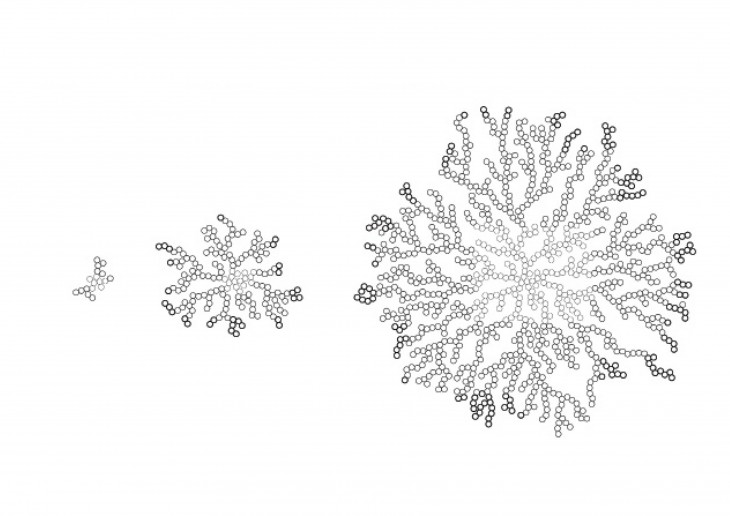
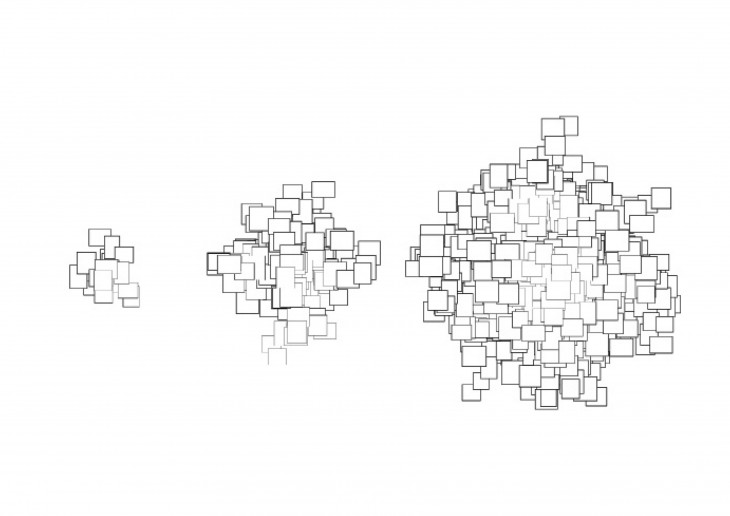
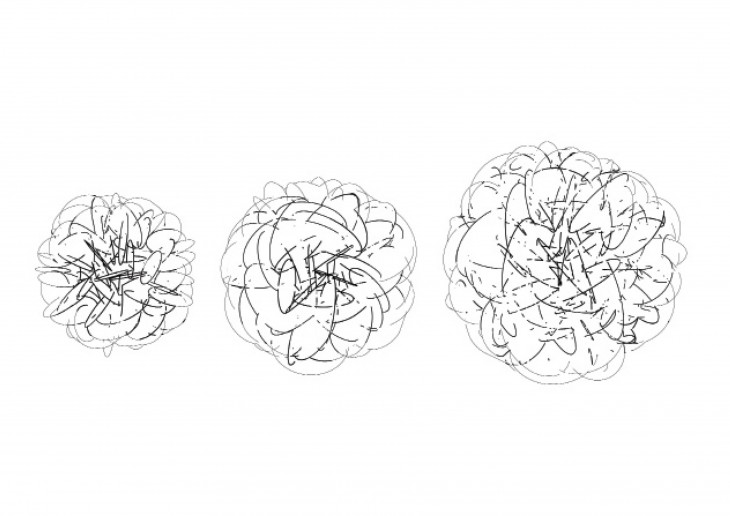
- They maintain their initial shape as the grow and just keep getting bigger in size.
Naturally on surfaces they sit on the bigger faces.
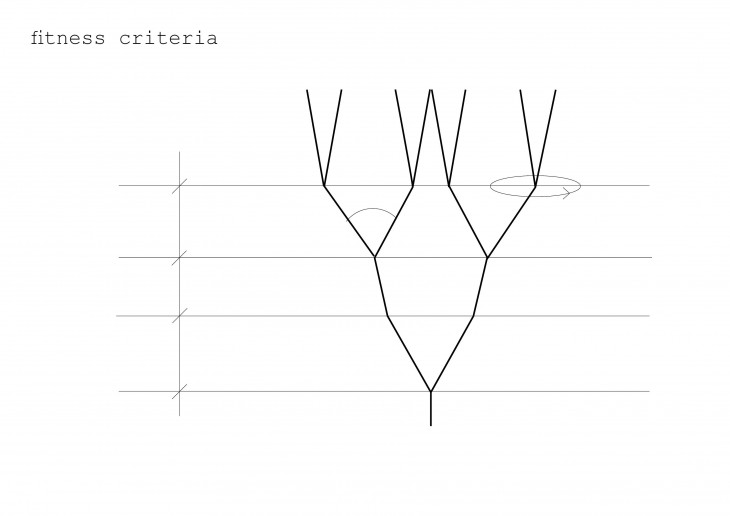
The shape of the crystal is based on what its made of and not on its size, so long as it is free to grow on all direction.
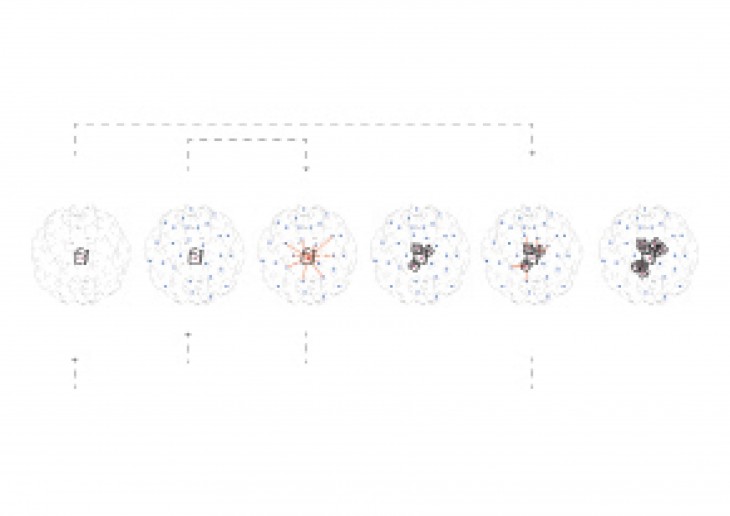
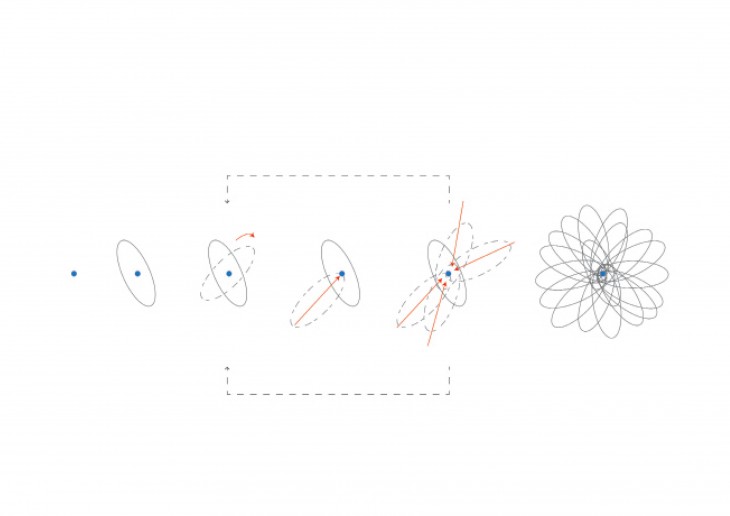
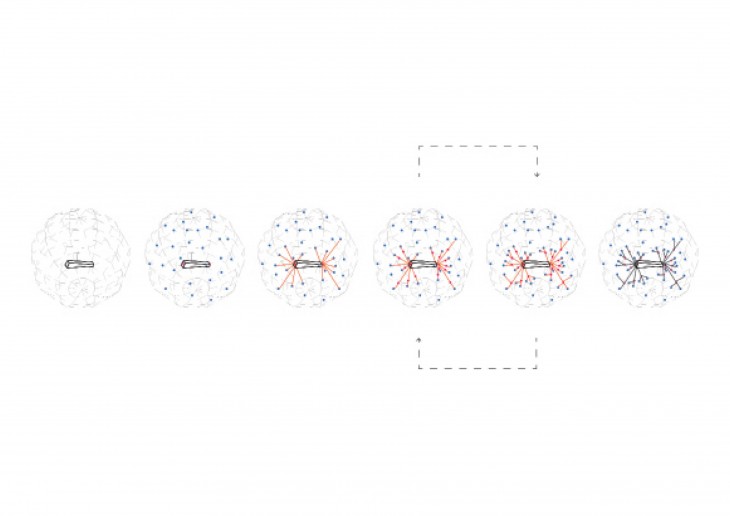
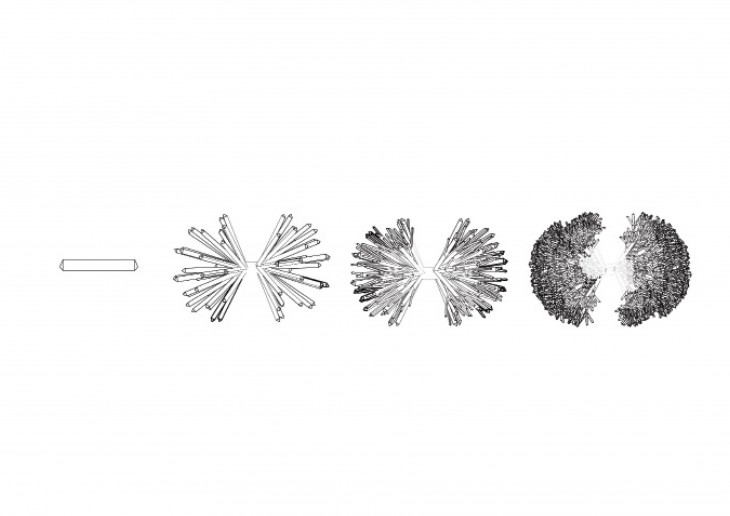
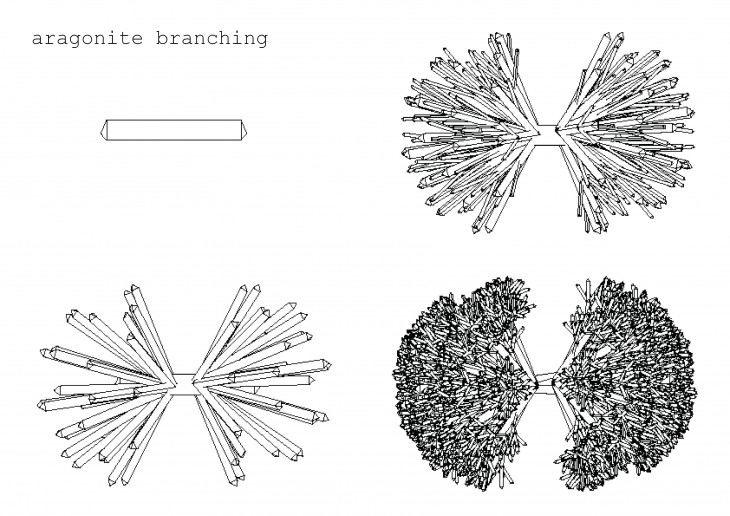
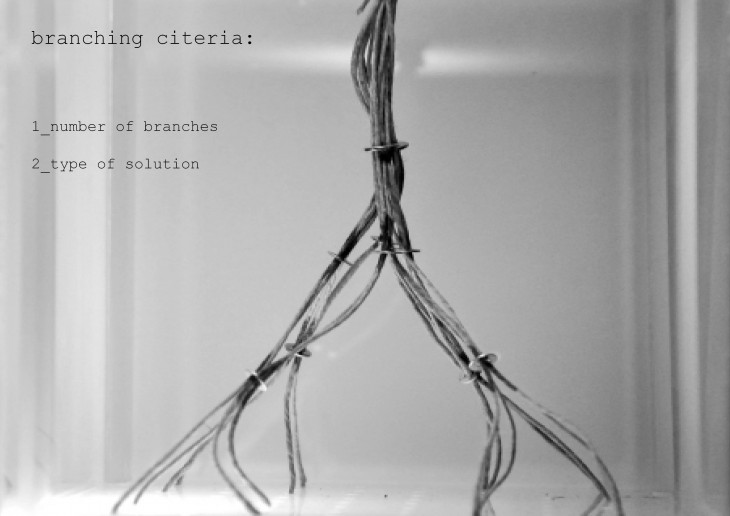
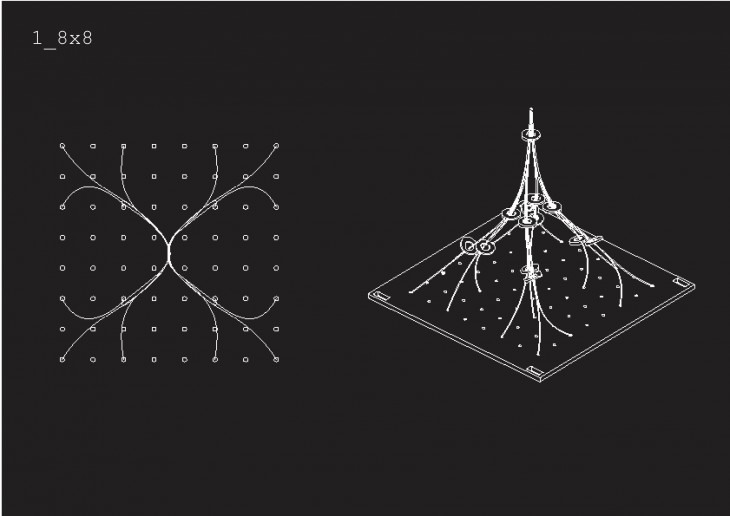
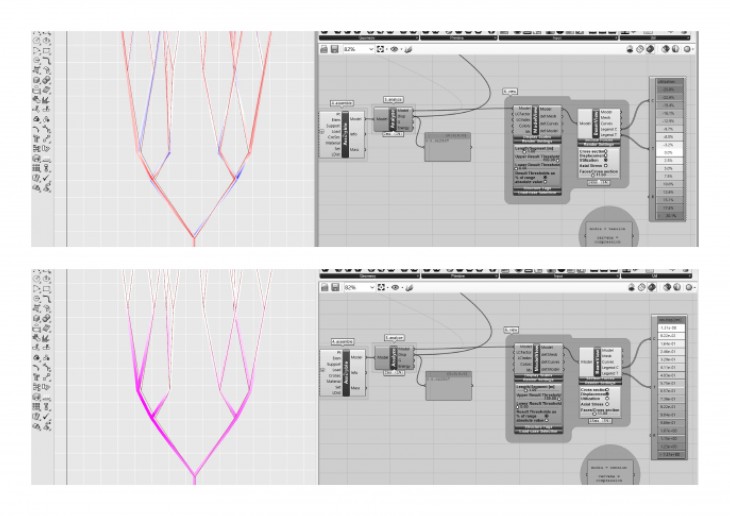
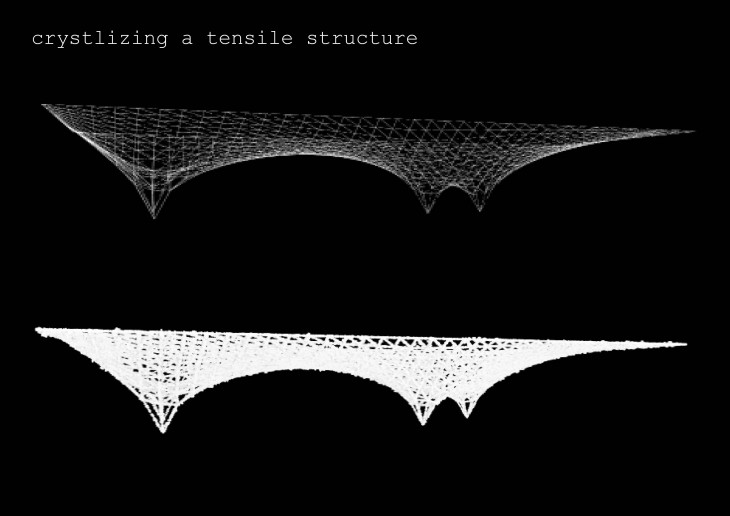
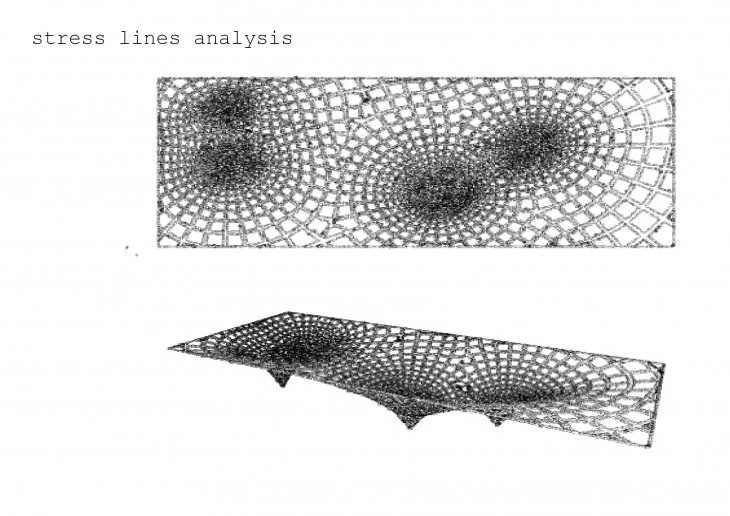
Crystals grow in different ways, from gases, water crystals such as snow flakes, and the most important way that how crystal grows is out of melted materials after cooling them down.
During crystallization, the individual crystals dont actually push their faces outwards. - The liquid next to the face gets solidified and adds itself on to the crystals face, and it does it in a way that the added liquid also forms a flat face so the crystal keeps its shape while it grows.


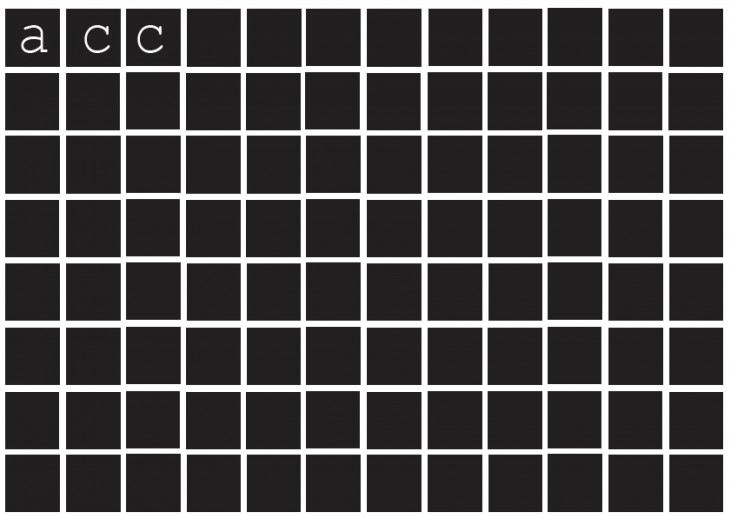
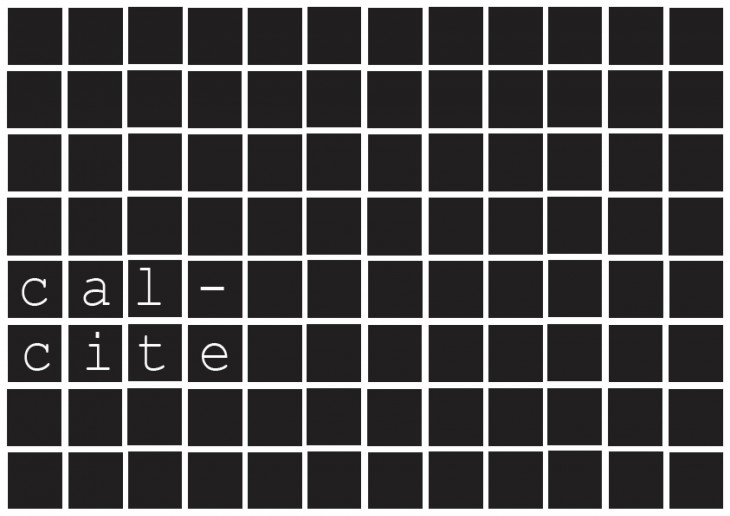
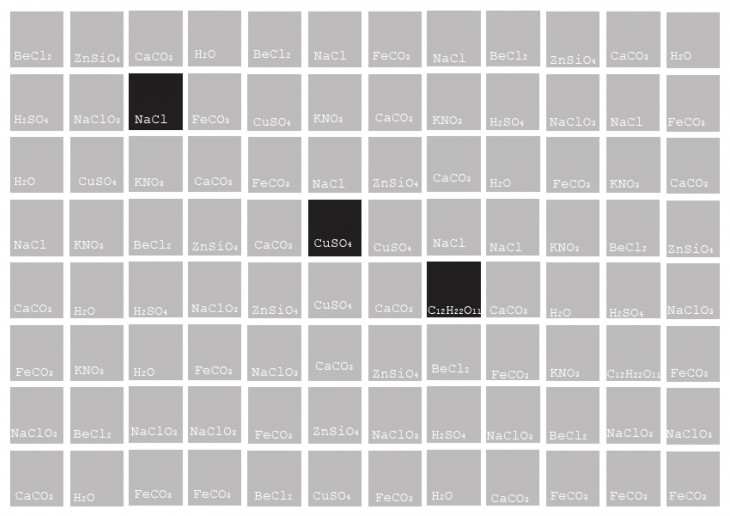
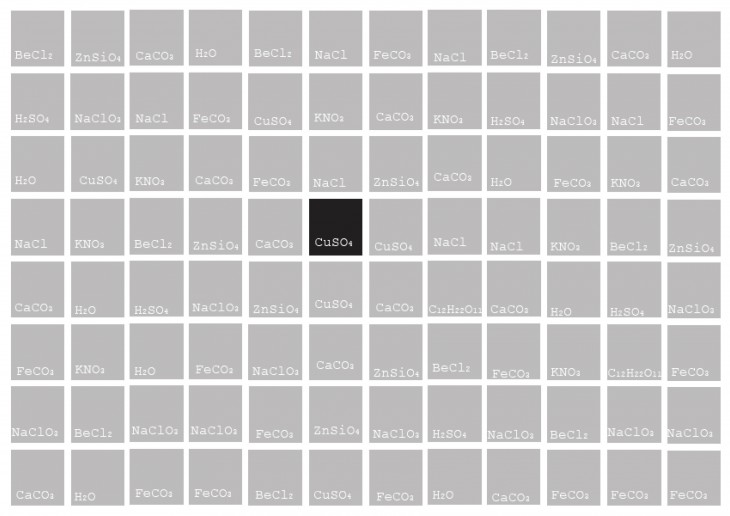
Why CaCo3 ?
Calcium Carbonate is classified as one with the highest contribution to the Eco-system.
Almost every product in our daily lives either contains calcium carbonate or has some association with the mineral during its production.
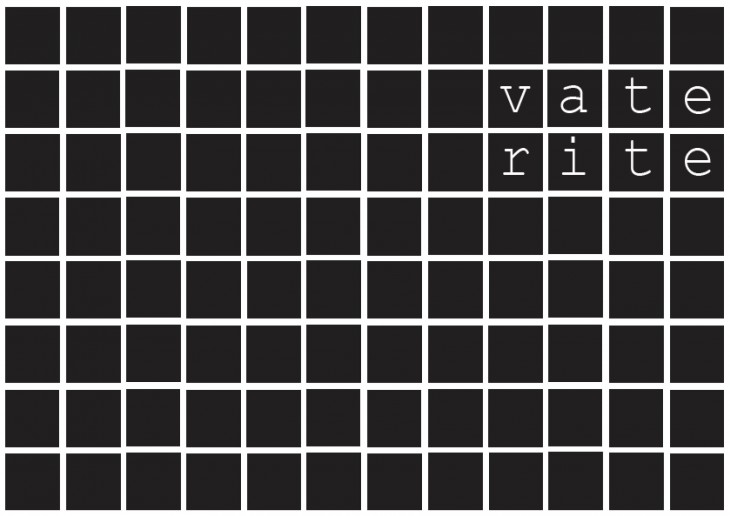
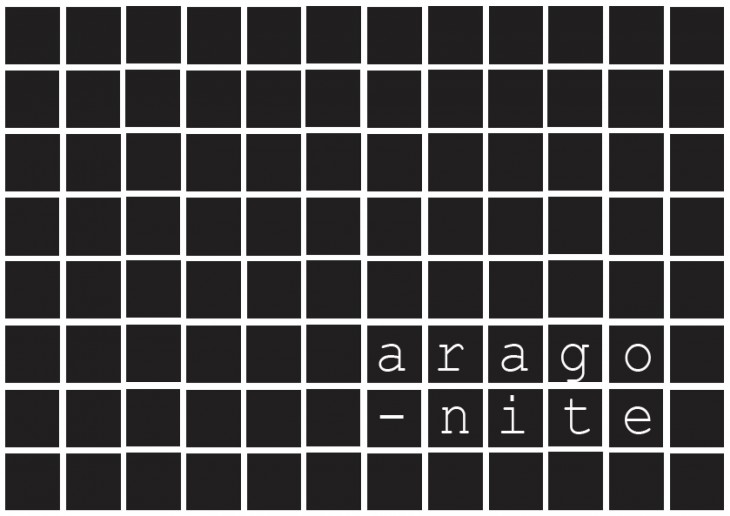
As a result, the four calcium carbonate mineral forms- ACC, calcite, aragonite and vaterite – are among the most important rock-forming minerals.
These have a crystalline structure and exhibit structural formations with distinct characteristics and Morphological properties.




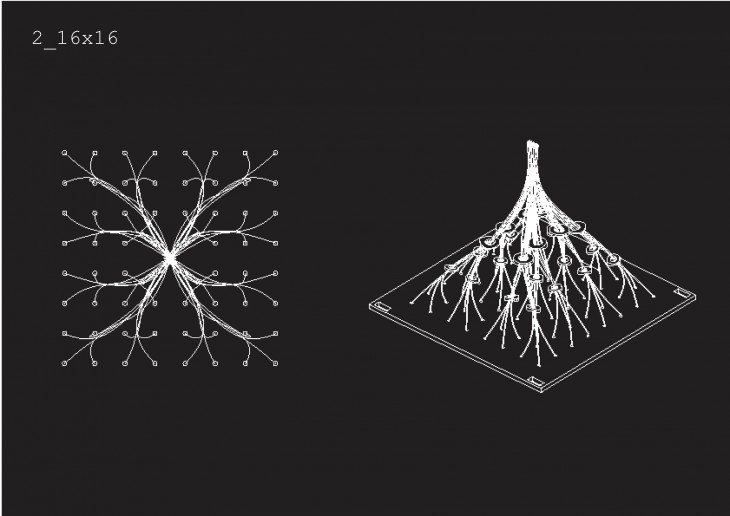
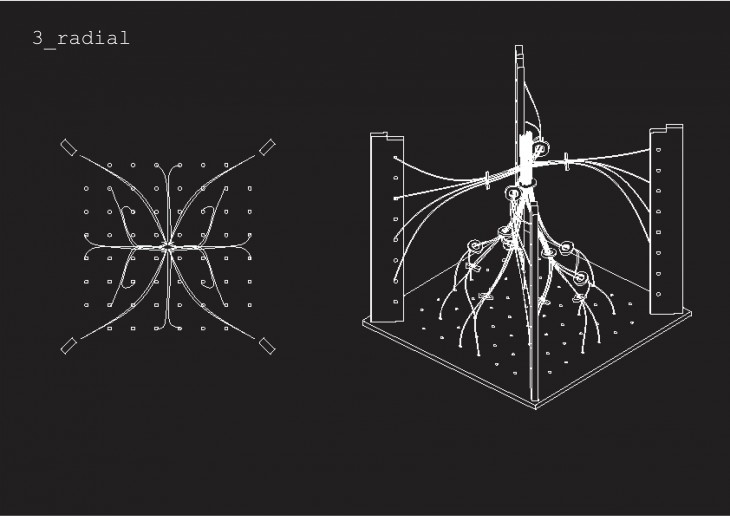




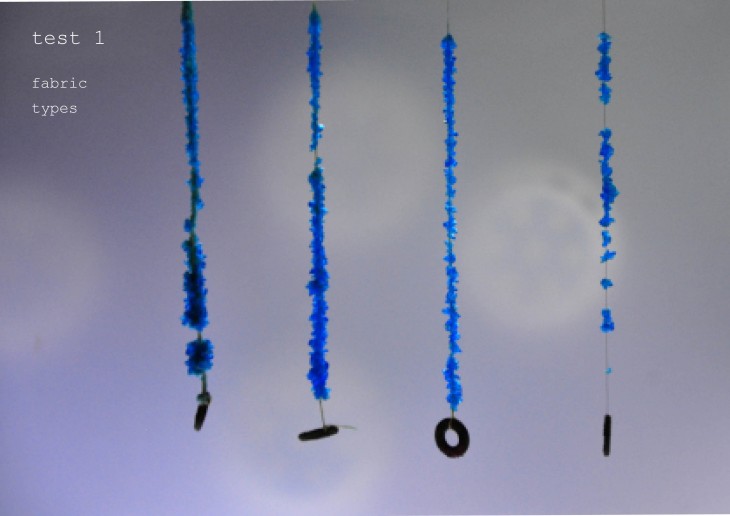
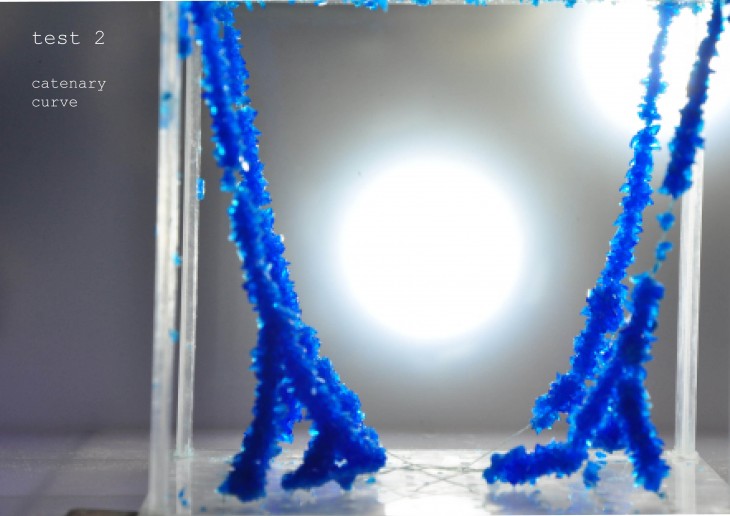

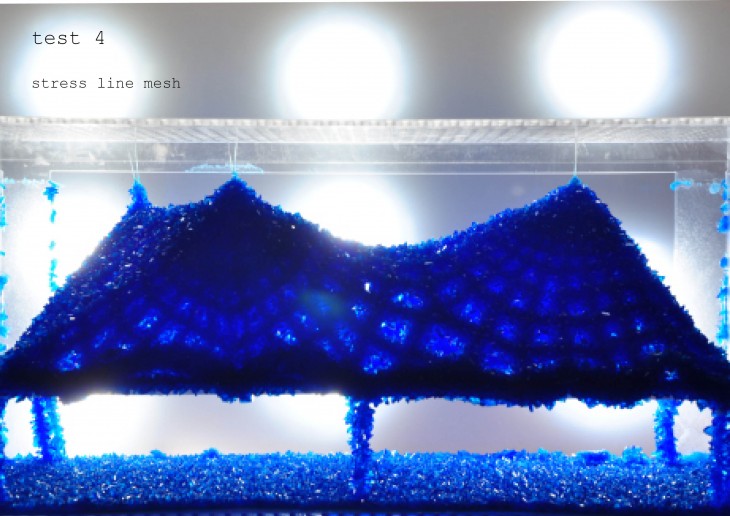

Crystallization is a project of IaaC, Institute for Advanced Architecture of Catalonia developed at Master of Advanced Architecture in 2015/16 by:
Student:
- Nisarg sheth
- Elena janeva
- Viplav goverdhan
- Tanuj Thomas
- Inthat ueasak-aree
- Jakub havlik
Faculty:
- Carmello Zappulla
- Claudia Pasquera
- Maria kuptsova